In cooling tower systems, cycles of concentration in cooling towers play a critical role in determining both operational efficiency and water usage. Managing this parameter properly ensures that the cooling system performs optimally while minimizing scaling, corrosion, and biological fouling.
For industries that rely heavily on cooling systems, such as power plants, refineries, and manufacturing facilities, understanding and controlling the cycles of concentration (COC) is essential to achieving sustainable, cost-effective operations.
What Are Cycles of Concentration in Cooling Towers?
The term cycles of concentration (COC) refers to the ratio of the concentration of dissolved solids (such as minerals and salts) in the cooling tower water compared to the concentration in the makeup water.
When water circulates through a cooling tower, a portion of it evaporates to remove heat from the system. However, while pure water evaporates, the dissolved minerals remain behind, increasing their concentration in the circulating water. Over time, this leads to higher levels of total dissolved solids (TDS), hardness, chlorides, and other minerals.
The cycles of concentration quantify how concentrated these impurities become. In simple terms:
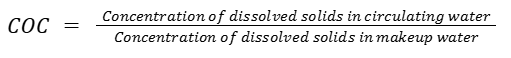
For example, if the TDS in the circulating water is 1,000 mg/L and the TDS in the makeup water is 250 mg/L, the COC is 4. This means the dissolved solids have concentrated four times compared to the makeup water.
A higher COC indicates greater water efficiency because more water is being reused before being discharged as blowdown. However, too high a COC increases the risk of scaling and corrosion. On the other hand, lower COC reduces these risks but wastes more water due to excessive blowdown. The challenge lies in finding the optimal balance.
How to Calculate Cycles of Concentration
The calculation of cycles of concentration in cooling towers can be performed in several ways, depending on available data. Below are the common methods used in industrial water treatment.
1. Based on Conductivity
Conductivity is often used as a quick indicator of total dissolved solids. The formula is:
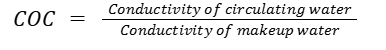
This is one of the simplest and most frequently used methods because conductivity meters are easy to install and continuously monitor.
Example:
- Conductivity of makeup water = 400 µS/cm
- Conductivity of circulating water = 1,600 µS/cm
COC = 1600/400= 4
This means the water in the system has gone through four cycles of concentration before part of it is discharged.
2. Based on Chloride or Silica Concentration
When conductivity data is unavailable or unreliable due to chemical additions, chloride or silica levels can be used instead because they are typically conservative ions that do not react within the system.
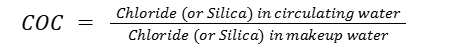
For instance:
- Chloride in makeup water = 50 mg/L
- Chloride in circulating water = 200 mg/L
COC = 200/50= 4
Both chloride and silica are preferred parameters when specific treatment chemicals interfere with conductivity readings.
3. Using Blowdown and Evaporation Data
In systems where flow rates are measured, COC can also be determined from the balance between evaporation and blowdown.
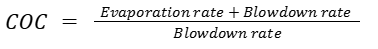
If evaporation is 10 m³/h and blowdown is 2.5 m³/h:
COC = (10 + 2.5) / 2.5= 5
This approach is more accurate in large industrial setups where water flow data is closely monitored.
Read Also: How to Reduce Boiler Blowdown for Operational Efficiency?
Typical COC Ranges and Their Implications
| Type of Cooling Tower Water | Typical COC Range | Remarks |
| Soft or treated water | 5 – 7 | Can achieve higher COC due to low scaling potential |
| Moderate hardness water | 3 – 5 | Common for most industrial cooling towers |
| Hard or high TDS water | 2 – 3 | Requires chemical treatment to increase COC safely |
| Seawater cooling towers | 1.5 – 2 | Limited by high chloride concentration |
These ranges are not absolute. The actual allowable COC depends on factors such as the makeup water quality, operating temperature, chemical treatment program, and cooling tower material.
Why Managing Cycles of Concentration Is Important
Proper management of cycles of concentration in cooling towers affects both system performance and operational costs. Below are the key benefits of controlling COC effectively:
1. Water Conservation – Higher COC reduces blowdown frequency, minimizing water waste.
2. Energy Efficiency – Maintaining optimal COC helps sustain efficient heat transfer and cooling performance.
3. Reduced Chemical Costs – Proper COC control ensures that water treatment chemicals are used efficiently.
4. Minimized Scaling and Corrosion – Balanced cycles prevent excessive mineral buildup and metal degradation.
5. Environmental Compliance – Reduced discharge means lower environmental impact and easier compliance with wastewater regulations.
How to Optimize Cycles of Concentration
Optimizing cycles of concentration in cooling towers requires a combination of accurate monitoring, water treatment chemistry, and system design improvements. The goal is to reach the highest possible COC without triggering scaling, corrosion, or biological fouling.
1. Control Scaling
Scaling occurs when dissolved minerals, primarily calcium carbonate or silica, precipitate out of the water and form deposits on heat exchange surfaces. To control scaling:
- Use scale inhibitors such as phosphonates or polymers.
- Maintain proper pH to keep minerals soluble.
- Monitor Langelier Saturation Index (LSI) to predict scaling tendency.
- Install side-stream filtration to remove suspended solids that act as nucleation points for scale formation.
By managing scaling potential, systems can safely operate at higher COC.
2. Prevent Corrosion
Corrosion can occur when water becomes too aggressive, especially at low COC or when pH is poorly controlled. To prevent corrosion:
- Use corrosion inhibitors (phosphate-based or molybdate-based).
- Maintain pH in the recommended range (typically 6.5–9, depending on materials).
- Regularly inspect and clean condenser tubes and tower basins.
- Avoid high chlorides or sulfates that attack metal surfaces.
Balancing the water chemistry helps prolong equipment lifespan and supports stable COC.
Read Also: Corrosion Inhibitor: Maximum Protection for Your Cooling System
3. Control Biological Fouling
Microbial growth, including algae and bacteria, can thrive in cooling towers due to warm and nutrient-rich conditions. Biofouling reduces heat transfer and can produce corrosive by-products. Effective control includes:
- Regular dosing of biocides and biodispersants.
- Maintaining residual disinfectant levels.
- Periodic cleaning of tower fill and basin.
- Monitoring microbial counts or using ATP testing for biological activity.
A clean system allows water to be cycled more times before requiring blowdown.
4. Monitor and Automate System Control
Automation plays a key role in maintaining stable cycles of concentration. Installing online sensors for conductivity, pH, and temperature allows automatic blowdown control and chemical dosing. These systems help maintain COC within the desired range and prevent deviations that could cause scaling or corrosion.
A well-calibrated controller system can automatically adjust blowdown valves when conductivity exceeds a set limit, ensuring that the system remains within the optimal concentration ratio at all times.
5. Optimize Chemical Treatment Programs
The correct combination of chemical treatments can push the system to achieve higher COC safely. The chemical program may include:
- Scale inhibitors to prevent precipitation.
- Corrosion inhibitors to protect metal surfaces.
- Biocides for microbial control.
- pH adjusters to maintain water chemistry stability.
Partnering with an experienced water treatment provider ensures that the chemical treatment is tailored to the specific water source, operating conditions, and system design.
6. Evaluate Water Source and Pretreatment
If the makeup water has high hardness or TDS, it limits the achievable COC. Pretreatment methods such as softening, reverse osmosis, or filtration can significantly improve water quality and allow higher cycles, resulting in lower water consumption and operational cost.
Achieving the Right Balance
While higher cycles of concentration improve water efficiency, there is a practical limit. Operating beyond the ideal point increases the risk of scaling and fouling, leading to higher maintenance costs and downtime. The optimal COC must be determined through comprehensive water analysis, chemical control, and system monitoring.
An experienced water treatment specialist can determine the most cost-effective and sustainable balance between water savings and system protection.
Read Also: Common Cooling Water Tower Problems and Solutions
Optimize Your Cooling Tower Performance with Lautan Air Indonesia
Managing cycles of concentration in cooling towers is not only about maximizing water reuse but also about ensuring long-term system reliability. Achieving the ideal COC requires a precise understanding of water chemistry, regular monitoring, and the right combination of chemical treatment and automation.
Lautan Air Indonesia, with more than four decades of expertise in industrial water treatment, offers comprehensive solutions to help industries optimize cooling tower performance. From chemical supply (scale, corrosion, and biocide treatment) to monitoring systems, filtration, and operation & maintenance services, Lautan Air Indonesia provides everything needed to maintain efficient, sustainable, and compliant cooling operations.
If you are looking to enhance cooling tower efficiency, reduce operational costs, and extend equipment lifespan, contact with Lautan Air Indonesia today to design a customized water treatment program that fits your system needs.



